Infertility
News about the blastocyst
The blastocyst corresponds physiologically to the last embryonic stage before implantation.
Theoretically, after fertilization in the trumpet, the embryo continues its development while parsing the trumpet to reach the uterine cavity around 4-5th day, at the blastocyst stage, to settle there.
At post-fertilization J1, the newly formed embryo was zygote and contained 2 haploid pronucleus, cell structures corresponding to each of the two nuclei, one from the sperm and the other from the oocyte. At the 25th post-fertilization hour, the embryo began its cell division. It contains 4 cells at J2 and 8 cells at J3. On the 4th day he is in the morulæ stage. It reaches the blastocyst stage on the 5th day and then breaks on the 6th day before entering the uterine cavity.
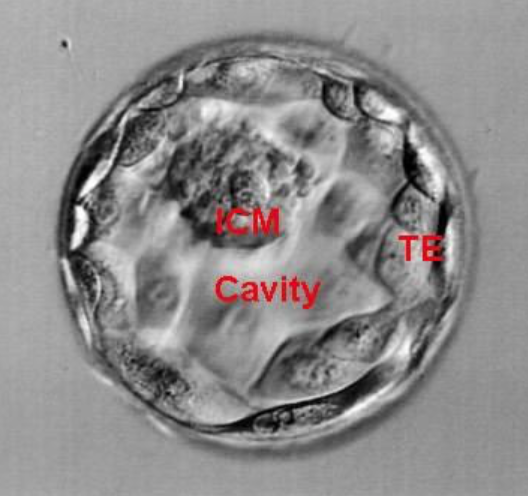
The blastocyst consists of the blastocele, the internal cell mass (MCI) that originated the fetus, and the trophectoderm (TE) that produced the placenta (Figure 1).
In vitro, blastocyst is evaluated according to the Gardner and Schoolcraft classification (tables). This gives 3 quality scores based on the stage of development in blastocyst (embryonic expansion and natural hatching status) (score 1-6), the quality of the internal cell mass (score A-C) and the quality of the trophectoderm (score A-C) (figures 2-9).
Numerous studies such as Goto et al.(1) show that in addition to the age of the woman, there is a significant correlation between blastocyst quality and the rate of clinical pregnancies, the rate of evolutionary pregnancies and the rate of births.
Expansion grade |
Stage of embryonic development |
1 |
Blastocle less than 50% of embryonic volume |
2 |
Blastocle greater than 50% of embryonic volume |
3 |
Blastocle completely filling the embryo, blastocle unexpanded |
4 |
Expanded blast, thinning of pellucid zone |
5 |
Blastocle in hatching |
6 |
Blastocèle hills |
MCI Grade |
Quality of internal cell mass |
A |
Numerous cells, well compacted |
B |
Several cells, roughly grouped |
C |
Very few cells |
Grade TE |
Trophectoderm quality |
A |
Numerous cells forming a festooned epithelium |
B |
Few cells forming a loose epithelium |
C |
Very few cells forming a smooth epithelium |
Tables: Classification of Gardner and Schoolcraft.
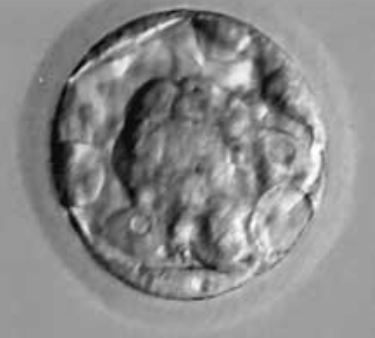
Figure 2: Blastocyst B1.
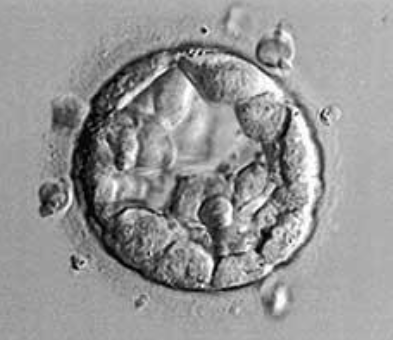
Figure 3: Blastocyst B2BB.
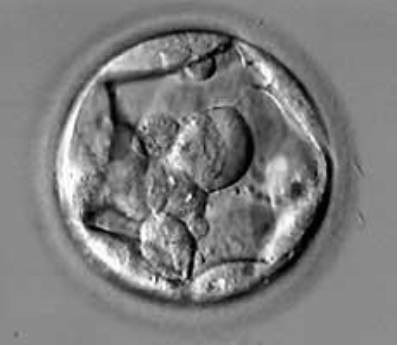
Figure 4: B2BC Blastocyst.
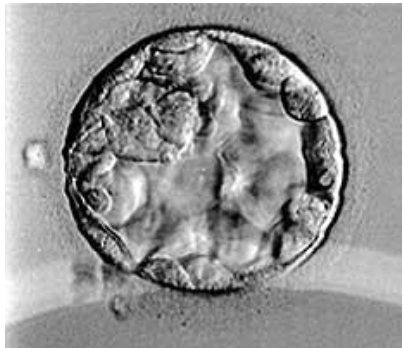
Figure 5: Blastocyst B3BB.
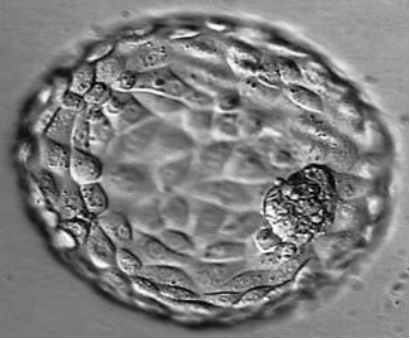
Figure 6: Blastocyst B4AA.
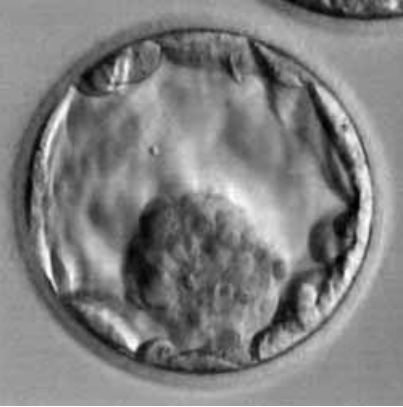
Figure 7: Blastocyst B4AB.
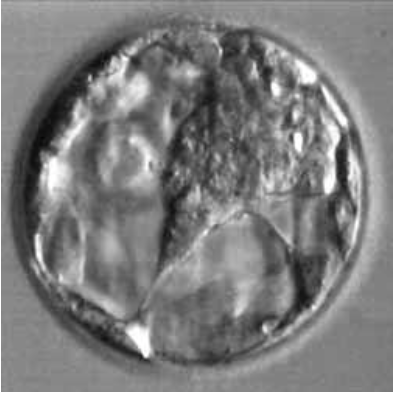
Figure 8: Blastocyst B4BB.

Figure 9: Blastocyst B5.
The goal of the AMP is to achieve a single pregnancy leading to the birth of a healthy child. However, the difficulty lies in obtaining and choosing the embryo that can achieve this optimal result. Given the pitfalls of embryonic culture, the majority of MPA centers in France transfer J2-J3 split embryos based on multiple embryonic classifications. The purpose of these classifications is to select the embryo with the best implant potential. The cleaved embryo was previously considered to be the best choice for transfer, as well as for embryonic freezing, the slow freezing technique yielding better results on the cleaved embryos than on the blastocysts.
However, with the rapid progress of the culture media and embryonic vitrification, transfer and freezing to the blastocyst stage present many advantages.
Indeed, many studies (Schoolcraft and Gardner(2,3)) have shown the value of transferring to the blastocyst stage rather than to the cleaved embryo stage in terms of implantation rate and pregnancy rate.
In addition to improved results, prolonged embryo culture would allow natural selection of embryos, since it extends after activation of the embryonic genome occurring at J3: for example, embryos capable of forming in vitro blastocysts would correspond to embryos with the greatest implant potential (Martins et al.(4)).
In AMP, implantation depends not only on embryonic quality but also on endometrial receptivity. Theoretically, blastocysts would have a greater implant potential as embryos reach the uterus in vivo about 4th day after fertilization; for example, in AMP, transferring embryos later, in terms of exposure of the embryo to the uterine environment, would be equivalent to a natural cycle. Indeed, long considered the most optimal environment for embryos, the uterine cavity may in some cases be a hostile environment. The supra-physiological rate of estradiol due to ovarian stimulation increased uterine contractions and altered endometrial receptivity, sometimes associated with a concomitant increase in progesterone rate (Fanchin et al.(5)). The transfer of blastocysts could prevent premature exposure to a hostile uterine environment.
Despite the advantages of transfer to the blastocyst stage, there are some disadvantages to prolonged cultivation. Although the meta-analysis of Papanikolaou et al.(6) shows a significantly higher rate of live births after the transfer of fresh blastocysts compared to the transfer of cleaved embryos, with an equal number of transferred embryos, these results remain controversial since other studies (Martins et al. 2017(4)) do not could confirm that.
Another disadvantage of protracted culture is that the in vitro embryonic environment with culture media would be less beneficial than the in vivo environment. The prolonged culture after activation of the embryo genome could on the contrary be deleterious to the embryo, leading to the failure of blasulation of the cultured embryos and thus to the absence of transfer of embryos that could have been implanted had they been transferred to the stage of the J2/J3 split embryos. Guérif et al.(7) evaluated the effectiveness of the transfer of a blastocyst in women under 36 years of age compared with the transfer of two cleaved embryos. In this study, the transfer rate was significantly lower in single blastocyst transfer couples compared with those transferred from 2 cleaved embryos (88% versus 100% respectively).
The transfer of blastocysts(8) and Papanikolaou et al.(6) shows that the transfer of blastocysts is significantly associated with a higher rate of transfer failure due to blastoulation failure and a decrease in the rate of frozen embryos compared with the cleaved embryos. Papanikolaou analyzed the results of 8 studies on this data: the rate of culture failure and thus no transfer was approximately 9.5% in the blastocyst group versus 4.3% in the cleaved embryo group.
An increase in the length of the culture corresponds to an increase in the workload at the laboratory.
Literature Review: site status
In view of the protracted culture’s advantages and disadvantages, this state of the knowledge on blastocysts will enable us to balance the benefit and risk of this technique with the aim of adapting our daily practices to AMP.
Improved cultivation methods
Many variables affect the success of an AMP attempt. Transferring a euploid embryo is the result of a complex system that requires many prior steps. Embryonic culture and manipulation of the embryo in the laboratory can affect its implant potential. Environmental stress factors due to culture environments and culture conditions can have a biochemical, metabolic and epigenetic impact (Swain et al.(9)). For this reason, a continual improvement of knowledge allows for an improvement of the culture environments in order to optimize embryonic development up to the blastocyst stage and increase the number of good quality embryos.
In vivo, embryos are exposed to oxygen concentrations of 2% to 8%. In vitro embryonic culture, using oxygen levels at atmospheric pressure, alters embryo metabolism and blastocyst development in many animal species. Thus, better birth rates after blastocyst transfer were achieved by lowering the oxygen concentration in the incubators (Wale et al.(10); Calzi et al.(11)).
Prolonged embryo cultivation to the blastocyst stage should therefore be carried out in hypoxia, hence the need for adequate equipment in the laboratory, i.e. three-gas ovens to achieve a decrease in oxygen concentration.
eSET Policy
Although the first post-IVF pregnancy occurred after the transfer of a single embryo, this practice of transferring only one embryo was quickly replaced by the transfer of several embryos, which allowed for a higher rate of pregnancies. However, this increase was accompanied by an increase in the rate of multiple pregnancies. The one-embryo transfer policy (eSET) was therefore referred to as a strategy to reduce multiple pregnancy rates after AMP.
The study by Zander et al.(12) shows that delaying transfer to the blastocyst stage rather than transferring to the traditional cleaved stage allows for better selection of the embryo most likely to implant, maximizing the chances of pregnancy, even with the transfer of a single embryo. This blastocyst eSET policy can therefore reduce the rate of genital pregnancies while maintaining a rate of deliveries close to that of a transfer of two cleaved embryos.
A randomized controlled prospective study conducted by Papanikolaou in 2006(13) was conducted to determine whether there are differences in pregnancy and birth rates between women with J3 cleaved embryo transfer and women with J5 blastocyst transfer. It shows a significant increase in the rate of pregnancies in the blastocyst group. Birth rates were significantly higher in the blastocyst group.
Transfer to the blastocyst stage therefore increases the rate of pregnancy per transferred embryo, which is particularly relevant in an eSET policy context in order to reduce multiple pregnancies.
Improved results
A recent Cochrane metaanalysis of 2016(8) shows that blastocyst transfers have higher rates of clinical pregnancy and live births compared to transfers of fresh cleaved embryos. However, the fundamental bias of many studies analyzed in this metaanalysis was to include groups of patients who had an unequal number of transferred embryos, with more embryos transferred to the private stage than to the blastocyst stage.
The meta-analysis of Papanikolaou et al.(6) compared studies with a primary inclusion criterion of an equal number of embryos transferred into the blastocyst transfer group and the cleaved embryo transfer group. It shows a significant increase in the rate of live births and the rate of clinical pregnancies during blastocyst transfers.
Nevertheless, the important bias of these studies is that they report results only in fresh transfer, while only the cumulative rate of pregnancy including fresh and frozen transfers reflects the results of a cycle.
Embryonic freezing and cumulative pregnancy rate
The introduction of vitrification as a freezing technique revolutionized the survival rates of cryopreserved embryos and improved pregnancy rates. Thus, the main indicator for comparing blastocysts and cleaved embryos should be the cumulative rate of pregnancies.
A first study in 2013 (Zhu et al.(14)) shows a significant increase in the rate of pregnancy by cycle and transfer and in the rate of implantation from the first cycle of frozen embryo transfer during blastocyst transfers compared to the cleaved embryos. However, the cumulative rate of pregnancies is similar in both groups.
The study by De Vos et al.(15) in 2016 confirms that the rate of live births per cycle is significantly higher after the transfer of a fresh blastocyst. Similarly, the cumulative rate of pregnancies is the same after transfer of blastocysts or cleaved embryos, but pregnancy is obtained more quickly after transfer of blastocysts, as the number of embryos required to obtain pregnancy is significantly less for blastocysts.
The Carvalho et al.(16) study in 2017 and Guérif et al.(17) in 2009 confirm these findings.
Prolonged culture as an embryonic selection tool allows patients to reach pregnancy faster than with J2-J3 culture, but does not increase the cumulative chances of pregnancy. Prolonged cultivation of supernumerary embryos for cryopreservation would optimize the time taken care of.
Blastocyst interest in case of an extra uterine pregnancy
The study by Fang et al.(18) shows that the risk of extra-uterine pregnancy is significantly lower in the case of transfer to the blastocyst stage compared to the transfer of J3-cleaved embryos. These results would likely be explained by the decrease in uterine contractility to near-quiescent status at transfer time (Fanchin et al.(5) and the wide diameter of the blastocyst (Schoolcraft et al.(19)). It shows that a transfer of frozen blastocysts is associated with a significantly lower level of GEU than in the case of a transfer of fresh blastocysts.
Repeated implantation failures and embryonic quality of split embryos
The study by Karacan et al.(20) suggests that in patients, after repeated failure of implantation in AMP, there would be no benefit to transfer to the blastocyst stage. Nevertheless, there are many biases in this study. Other studies prior to this study had shown interest in transferring to the blastocyst stage in patients who had experienced multiple IVF failure.
The retrospective study of Cruz et al.(21) shows an increase in implant and pregnancy rates with blastocyst transfer compared to the J3 embryo transfer in patients with multiple IVF cycle failures. Guérif et al.(22) compare blastocyst transfer to J2 embryos in patients who had at least 2 transfer cycles. It shows higher rates of implantation and clinical pregnancies with blastocyst transfer.
Similarly, in couples with previous IVF failures due to the absence of good quality embryos, the study by Guérif et al. in 2011(7) shows the value of protracted culture in these couples. Delaying transfer and leaving these embryos in prolonged culture allows to select the embryo to be transferred and to transfer only if blastocyst formation.
Blastocyst and euploidy
Although some genetically abnormal embryos are capable of reaching the blastocyst stage, the risk of aneuploidy of a good quality blastocyst is less important than a good quality cleaved embryo. The study of Capalbo et al.(23) shows that only the morphology of blastocysts is predictive of the embryo’s chromosome status, with the euploidy rate correlated with the quality of blastocysts according to the Gardner and Schoolcraft classification. The diagnosis of complex aneuploidy (multiple chromosome anomalies) is associated with the morphology of blastocysts. The study of Alfarawati et al.(24) shows a correlation between blastocyst morphology and aneuploidy, suggesting a negative effect of aneuploidy on blastocyst development, internal cell mass, and trophectoderm. Low-grade blastocysts have an increased incidence of monosomy and chromosomal abnormalities.
A recent retrospective study by Gaurav Majumdar et al.(25) suggests that euploidy rates are significantly higher for good morphology blastocysts, and that there is no association between the morphology of cleaved embryos and the euploidy rate. In addition, it was shown that embryos that had slower development and reached the blastocyst stage at J6 had significantly lower euploidy rates than those that had faster development and achieved full expansion at J5. The implantation rate is identical for all euploid blastocysts, regardless of morphology or day of development (J5 or J6). However, there is a decrease in results with late blastocysts, i.e. those that reach the expanded blastocyst stage at the 6th day post-fertilization. This could be attributed to a loss of synchronicity with the endometrium. The cryoconservation of these blastocysts thus optimizes their implantable potential (Franasiak, 2013(26)). Gaurav also shows that the rate of FCS of euploid blastocysts is lower than that of euploid cleaved embryos.
In total, the morphology of blastocysts is correlated with the euploidy rate: the better the blastocyst quality the less the risk of aneuploidy is. In addition, the euploidy rate for J5 blastocysts was significantly higher than for J6 blastocysts.
Blastocysts and obstetral and neonatal risk
A meta-analysis of 6 studies in 2014 (Dar et al.(27)) shows that in AMP, the risk of premature birth (between 32 and 37 SA) of single pregnancies is significantly higher after transfer of blastocysts compared to cleaved embryos. The risk of birth defects is also higher when blastocysts are transferred. There are no significant differences in the risk of premature birth before 32 SA and for small birth weights.
In 2016, a meta-analysis of 12 studies (Martins et al.(28)) showed that blastocyst transfers were associated with a higher risk of premature birth (< 37 SA and < 32 SA), fetal macrosomia and perinatal mortality, but less of fetal hypotrophy.
More recently, Wang et al.(29) confirmed in a meta-analysis of 12 studies that the risk of premature birth is greater after transfer of blastocysts versus cleaved embryos. No difference in the risk of premature birth was found for frozen embryos. It shows an increased risk of fetal macrosomia after blastocyst transfer and an increased risk of fetal hypotrophy or NCIU after transfer of cleaved embryos.
However, these results remain controversial, as other studies show no significant difference in obstetric or neonatal risks (Oron et al.(30)). The same is true of the Chambers et al.(31) study in 2015, which showed no difference in the risk of premature birth after transfer of blastocysts versus cleaved embryos. And for the retrospective study conducted by Li et al.(32), which found no significant difference in rates of premature birth and birth weight for single or gimal pregnancies.
The study of Bouillon et al.(33) determined whether there was a relationship between blastocyst morphology and obterior and perinatal risks. It shows that low-grade morphology of blastocysts is not correlated with major obstetric and neonatal risks.
In conclusion, prolonged cultivation up to the blastocyst stage allows for self-selection of embryos, better rates of clinical pregnancy and childbirth, and allows the transfer of embryos under more natural conditions. However, the main risk due to the deleterious effects of culture is a blastulation failure with fewer exploitable embryos (transfer and freezing).
Results
In view of the advantages and disadvantages of protracted culture, this literature review allows us to balance the effectiveness and risk of this technique with the aim of adapting our daily practices to AMP. Transfer to the blastocyst stage should therefore be preferred in eSET policy, in the case of a history of extra-uterine pregnancy and in repeated failure of implantation, using means of optimal culture (adapted medium and hypoxia, mainly). Genetically, in the absence of a current authorization in France for pre-implantation genetic tests, transfer of blastocysts should be preferred in the case of repeated miscarriages or in older patients, since only the morphology of blastocysts is correlated with the aneuploidy rate. The results of studies on obstetric and neonatal complications remain controversial. Transfers of blastocysts would be associated with a higher risk of premature birth, fetal macrosomia and perinatal mortality; however, these results have not been confirmed in other studies.
Conclusion
The culture of the embryo to the implantation stage is well known today. A recent review by Morris(34) shows that an English team has allowed the cultivation of human embryos up to the gastrulation stage, 14 days after fertilization; this would show that the human embryo has intrinsic organizational capabilities, without the need for the participation of maternal tissues. This discovery will allow a better knowledge of the implementation of these early stages of embryonic development in order to predict when developmental defects are likely to occur and thus prevent early miscarriages. These results will certainly bring new ethical challenges to human embryonic culture.
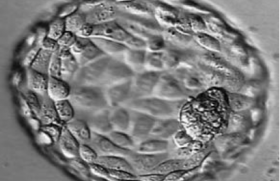
Vidéo 1 : Blastocyste B4AA.
Vidéo 2 : Blastocyste en éclosion.
References
Click on the references and access the Abstracts on

1. Goto S et al. Prediction of pregnancy rate by blastocyst morphological score and age, based on 1,488 single frozen-thawed blastocyst transfer cycles. Fertil Steril 2011 ; 95 : 948-52. Search the abstract2. Schoolcraft WB, Gardner DK. Blastocyst versus day 2 or 3 transfer. Semin Reprod Med 2001 ; 19 : 259-68. Search the abstract3. Gardner DK, Schoolcraft WB. Culture and trransfer of human blastocysts. Curr Opin Obstet Gynecol 1999 ; 11 : 307-11. Search the abstract4. Martins WP et al. Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstet Gynecol 2017 ; 49 : 583-91. Search the abstract5. Fanchin R et al. Uterine contractility decreases at the time of blastocyst transfers. Hum Reprod 2001; 16 : 1115-9. Search the abstract6. Papanikolaou EG et al. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis. Hum Reprod 2008 ; 23 : 91-9. Search the abstract7. Guerif F et al. Treating women under 36 years old without top-quality embryos on day 2: a prospective study comparing double embryo transfer with single blastocyst transfer. Hum Reprod 2011 ; 26 : 775-81. Search the abstract8. Glujovsky D et al. Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev 2016 ; CD002118. Search the abstract9. Swain JE et al. Optimizing the culture environment and embryo manipulation to help maintain embryo developmental potential. Fertil Steril 2016 ; 105 : 571-87. Search the abstract10. Wale PL, Gardner DK. The effects of chemical and physical factors on mammalian embryo culture and their importance for the practice of assisted human reproduction. Hum Reprod 2016 ; 22 : 2-22. Search the abstract11. Calzi F et al. Exposure of embryos to oxygen at low concentration in a cleavage stage transfer program: reproductive outcomes in a time-series analysis. Clin Lab 2012 ; 58 : 997-1003. Search the abstract12. Zander-Fox DL, Tremellen K, Lane M. Single blastocyst embryo transfer maintains comparable pregnancy rates to double cleavage-stage embryo transfer but results in healthier pregnancy outcomes. Aust N Z J Obstet Gynaecol 2011 ; 51 : 406-10. Search the abstract13. Papanikolaou EG et al. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos. N Engl J Med 2006 ; 354 : 1139-46. Search the abstract14. Zhu L et al. Blastocyst culture and cryopreservation to optimize clinical outcomes of warming cycles. Reprod Biomed Online 2013 ; 27 : 154-60. Search the abstract15. De Vos A et al. Cumulative live birth rates after fresh and vitrified cleavage-stage versus blastocyst-stage embryo transfer in the first treatment cycle. Hum Reprod 2016 ; 31 : 2442-9. Search the abstract16. Carvalho BR et al. Embryo stage of development is not decisive for reproductive outcomes in frozen-thawed embryo transfer cycles. JBRA Assist Reprod 2017 ; 21 : 23-6. Search the abstract17. Guerif F et al. Single Day 2 embryo versus blastocyst-stage transfer: a prospective study integrating fresh and frozen embryo transfers. Human Reprod 2009 ; 24 : 1051-8. Search the abstract18. Fang C et al. Frozen-thawed day 5 blastocyst transfer is associated with a lower risk of ectopic pregnancy than day 3 transfer and fresh transfer. Fertil Steril 2015 ; 103 : 655-61.e3. Search the abstract19. Schoolcraft WB1, Surrey ES, Gardner DK. Embryo transfer: techniques and variables affecting success. Fertil Steril 2001 ; 76 : 863-70. Search the abstract20. Meric Karacan et al. Comparison of the transfer of equal numbers of blastocysts versus cleavage-stage embryos after repeated failure of in vitro fertilization cycles. J Assist Reprod Genet 2014 ; 31 : 269-74. Search the abstract21. Cruz JR et al. Is blastocyst transfer useful as an alternative treatment for patients with multiple in vitro fertilization failures? Fertil Steril 1999 ; 72 : 218-20. Search the abstract22. Guerif F et al. Efficacy of blastocyst transfer after implantation failure. Reprod Biomed Online 2004 ; 9 : 630-6. Search the abstract23. Capalbo A et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod 2014 ; 29 : 1173-81. Search the abstract24. Alfarawati et al. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril 2011 ; 95 : 520-4. Search the abstract25. Gaurav Majumdar, BS et al. Relationship Between Morphology, Euploidy and Implantation Potential of Cleavage and Blastocyst Stage Embryos. J Hum Reprod Sci 2017 ; 10 : 49-57. Search the abstract26. Franasiak J et al. Investigating the impact of the timing of blasulation on implantation: active management of embryo-endometrial synchrony increases implantation rates. Fertil Steril 2013. DOI: 10.1016/j.fertnstert.2013.07.1710 Search the abstract27. Dar S et al. Neonatal outcomes among singleton births after blastocyst versus cleavage stage embryo transfer: a systematic review and meta-analysis. Hum Reprod 2014 ; 20 : 439-48. Search the abstract28. Martins WP et al. Obstetrical and perinatal outcomes following blastocyst transfer compared to cleavage transfer: a systematic review and meta-analysis. Hum Reprod 2016 ; 31 : 2561-9. Search the abstract29. Wang X et al. Comparative neonatal outcomes in singleton births from blastocyst transfers or cleavage-stage embryo transfers: a systematic review and meta-analysis. Reprod Biol Endocrinol 2017 ; 15 : 36. Search the abstract30. Oron G et al. Obstetric and perinatal outcome from single cleavage transfer and single blastocyste transfer: a matched case-control study. Gynecol Endocrinol 2015 ; 31 : 469-72. Search the abstract 31. Chambers GM et al. Risk of preterm birth after blastocyst embryo transfer: a large population study using contemporary registry data from Australia and New Zealand. Fertil Steril 2015 ; 104 : 997-1003 Search the abstract32. Li W et al. Blastocyst transfer is not associated with increased unfavorable obstetric and perinatal outcomes compared with cleavage-stage embryo transfer. Gynecol Endocrinol 2017 : 1-4. Search the abstract33. Bouillon C et al. Obstetric and perinatal outcomes of singletons aftersingle blastocyst transfer: is there any difference according to blastocyst morphology? Reprod Biomed Online 2017. pii: S1472-6483(17)30233-X. doi: 10.1016/j.rbmo.2017.04.009. [Epub Search the abstract34. Morris SA1.Human embryos cultured in vitro to 14 days. Open Biol 2017 ; 7. pii: 170003. Search the abstract